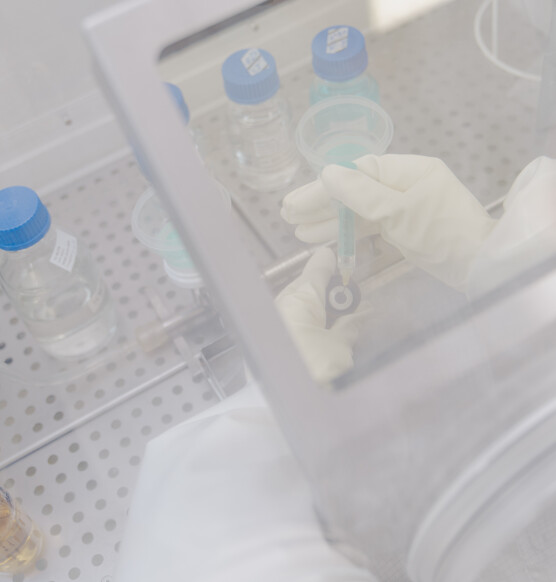
RADIOSTER.
Our division for the analysis of radioactive products.
View into radioster

Sterility testing
of radioactive products.
Reliable, precise and prompt testing for sterility via:
- Membrane filtration
Handling of high doses of radioactivity through patented process & patented isolator. It is safe, it is fast and clean.
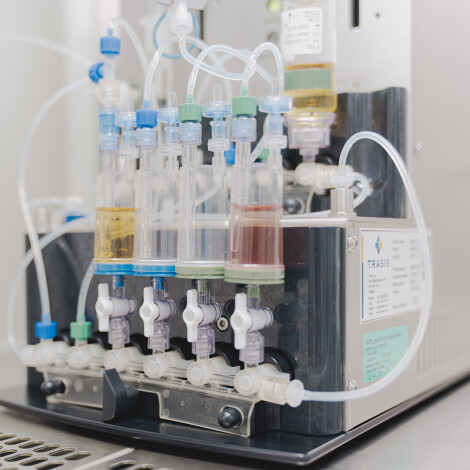
Validation
for the SteriNow®-cassette from Trasis.
Trasis has developed Sterinow® so that your products can be tested for sterility on site.
- No exposure
- No delay
- GMP and FDA compliant
We carry out product-specific validation for you so that you can present audit-proof documents.

Radiolabelling
of radioactive APIs.
We develop and control the radiolabelling of your active substances, including radiochemical purity, yield, and by-product profiling immediately after synthesis. Our integrated analytics enable rapid release despite short half-lives and ensure GMP-compliant documentation.

E&L- Studies
Analysis for Hot Products.
We identify and assess potential leachables from materials in radioactive formulations, including highly sensitive detection of critical compounds. These studies help minimize contamination risk and unwanted interactions.

Particle Testing (Visible & Sub-visible)
for Radiopharmaceuticals.
We quantify visible and sub-visible particles in your radioactive products to ensure compliance with quality and safety requirements. These measurements are specifically designed for hot matrices.

Nitrosamine Analysis
in Radiopharmaceuticals.
Using targeted methods, we identify and quantify nitrosamine-related risks directly in hot radiopharmaceutical products and materials. These analyses support regulatory safety and risk assessment.

Gas Chromatography (GC)
for Radiopharmaceuticals.
We analyze volatile compounds, residual solvents, and identity markers in radioactive products via gas chromatography, even at high activity levels. This supports your process and release analytics for radiopharmaceuticals.

Elemental Analysis (ICP-MS / AAS / AES)
in radioaktive samples.
Using advanced ICP techniques, we determine elemental and metal impurities in radioactive samples to confidently meet regulatory limits. Our analyses are optimized for the specific challenges of hot matrices.

HPLC Analysis
for hot Products.
Our HPLC methods deliver precise data on assay, purity, and degradation products in radioactive APIs and formulations. Analyses are validated for radioactive matrices and implemented in a GMP-compliant manner.